|
Case Report
The use of PRF in guided bone regeneration with xenograft around implants in a severe bone loss site: A case report
1 Dental Implant Specialist, Faculdade do Centro Oeste Paulista (Facop), Bauru, São Paulo, Brazil
2 Implantology Department Professor, Faculdade do Centro Oeste Paulista (Facop), Bauru, São Paulo, Brazil
3 Implantology Department Coordinator, Faculdade do Centro Oeste Paulista (Facop), Bauru, São Paulo, Brazil
Address correspondence to:
Nelson Leonel Del Hierro Polanco
Departamento de Farmacologia, FOB-USP, Vila Nova Cidade Universitária 17012901 - Bauru, SP,
Brazil
Message to Corresponding Author
Article ID: 100035Z07NP2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Polanco NLDH, Martins R, Bernini GF, Alonso F, Gennaro G. The use of PRF in guided bone regeneration with xenograft around implants in a severe bone loss site: A case report. J Case Rep Images Dent 2021;7:100035Z07NP2021.ABSTRACT
Introduction: Platelet-rich fibrin (PRF) is an autologous blood-derived biomaterial, which after a specific blood centrifugation process, results in a platelet-rich fibrin network and growth factors that are critical for bone formation and regeneration. The objective of this case report is to describe the use of PRF combined to a xenogen bone substitute for a guided bone regeneration technique (GBR) around implants.
Case Report: A patient with partial edentulism, presenting absence of the upper incisors, sought an implant-supported prosthesis in the area. An absence of adequate thickness of the maxilla was observed, after a clinical and radiographic evaluation. A GBR and immediate implant installation was planned; after anesthesia and total flap detachment, two Cone Morse implants were installed in the lateral incisors area, anchored only in a coronal and apical portion, presenting exposed threads in the middle and upper area due to a vestibular bone defect. The patient’s blood was collected and centrifuged for the PRF membranes and Sticky Bone preparation by combining iPRF and lyophilized bovine bone. The implants exposed threads were covered with the Sticky Bone and the PRF membranes to assist in the process of bone regeneration and surgical wound healing. Six months after the surgical procedure, an implant-supported fixed partial prosthesis was manufactured and both implants were osseointegrated and included in the patient’s rehabilitation.
Conclusion: We conclude that the use of PRF associated with bone substitutes contributed to bone regeneration, favoring the osseointegration process over a period of six months, enabling the patient’s aesthetic and functional rehabilitation.
Keywords: GBR, Implants, Osseointegration, PRF
Introduction
Reduced bone height and width are common limitations in dental implantology. To overcome these limitations, several techniques have been introduced, such as guided bone regeneration, alveolar ridge preservation, and sinus floor elevation [1].
Biomaterials are one of the bases in tissue engineering, bone grafts, scaffolds, and growth factors [2],[3],[4],[5],[6]. Considering their role, many biomaterials have been applied and suggested to use as an alternative to the autogenous bone, which is still the gold standard for bone augmentation [6],[7]. Aside from autogenous, xenogeneic, and allogeneic grafting materials, other natural and synthetic biomaterials have also been playing critical roles in many clinical cases [6],[8].
Deproteinized bovine bone (DBB) is an osteoconductive xenogeneic bone substitute that can withstand resorption during healing and can provide a good scaffold for natural bone growth. Deproteinized bovine bone can be used in combination with autogenous bone and its slow resorption properties could be an advantage in maintaining the volumetric stability of a bone graft [9],[10].
Platelet-rich fibrin (PRF) is the consequence of a whole blood centrifugation process, resulting on blood spontaneous coagulation, which, like in the natural blood clot, contains active platelets and leukocytes. Platelet-rich fibrin can be used by itself, compressing it to get a PRF membrane or mixing it with grafts and biomaterials [1].
It came as a more advantageous modification of the platelet-rich plasma technique, first used by Choukroun, which requires the addition of anticoagulants such as bovine thrombin during initial blood collection. The PRF is obtained simply by centrifugation without anticoagulants and is therefore strictly autologous [11],[12],[13].
Even though bone grafts nowadays come from different sources and materials, its stability is an important factor for the guided bone regeneration success, especially in a two-stage regeneration procedure. The graft stability is also desirable for dental implants in the osseointegration process and to ensure a good regeneration outcome [9],[14].
Collagen barrier membranes are commonly used during such procedures to ensure stability and avoid the migration of unwanted cells into the area. The combination or the replacement of these membranes with PRF may provide further regenerative advantages when compared to collagen barrier membranes alone. Although the results do not seem to confirm that PRF is better than other biomaterials, its ease of use, combined with its minimal costs and high success rates, makes it desirable [11].
The effects and uses of PRF are being summarized in many articles related to infrabony and furcation defects, extraction sockets, sinus lifting, gingival recessions, bone augmentation, tissue regeneration, and wound healing [1].
Platelet-rich fibrin is being applied to oral implantology due to its effects on hard and soft tissue healing process by the release of growth factors, obtaining satisfactory clinical results when mixed with bone graft around implants installed in bone defects [15].
Platelet-rich fibrin reduces local inflammation and promotes new bone formation, increasing cytokines and growth factors in the area. These growth factors are very important in cell differentiation, migration, and proliferation, leading to soft and hard tissue regeneration. These PRF membranes can also be used as a mechanical barrier, protecting open wounds and preventing infections [15].
This case report aims to demonstrate, through a clinical case, the use of PRF in addition to a xenogeneic bone substitute in the guided bone regeneration technique around implants.
Case Report
Patient SAPB, 47 years old, non-smoker, attended the Faculdade do Centro Oeste Paulista (FACOPH – Bauru, SP), requesting rehabilitation of the upper incisive region with implants and prior to the posterior areas for aesthetic reasons.
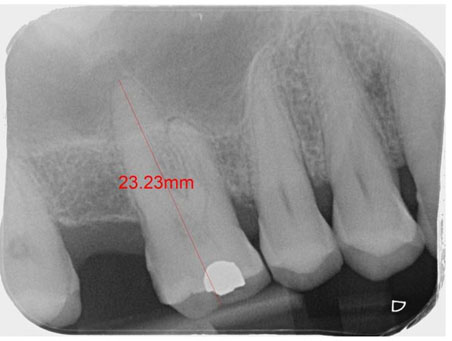
After clinical and radiographic evaluation, the oral surgeon found the need of implants installation in the anterior upper jaw, requiring horizontal bone augmentation (Figure 1).
Treatment plan
The installation of two drive 4.3 mm × 13 mm Cone Morse implants (Neodent), 2 mm below the bone crest, in the 12 and 22 element area (approximately 1.5 mm apart from the canines) was planned, with the objective of a fixed prosthesis that contemplates the missing elements.
Surgery
Infiltrative local anesthetic was performed with two 1: 100,000 Articaine tubes (DFL Articaine100) for the vestibular region and two 1: 100,000 Mepivacaine tubes (DFL Mepiadre100) for the nasopalatine foramen and palatal area. Modified Newmann incision was performed, maintaining the premolars papilla intact. Sterile saline in a 0.9% concentration was used during perforation for irrigation purposes and none was employed during implant installation.
After the installation of the implants with 45N of torque each, the large bone defect was visible leaving most implant surface uncovered (Figure 2).
Blood collection and centrifugation
To achieve bone formation in the bone defect area around implants, the surgeon collected the patient’s blood (4 tubes of 100 mL) from the median basilic vein and centrifuged two of them at a 2700 RPM protocol for 12 minutes to obtain the L-PRF (Figure 3). After separating them from the red cells segment, they were placed in a perforated tray for compression, resulting in L-PRF membranes (Figure 4).
The two resting tubes were also centrifugated at a 1650 RPM for 5 minutes to obtain the I-PRF and combine it with 1 g of bovine bone substitute (Bioinnovation materials – Bonefill) for the sticky bone preparation (Figure 5).
Grafting
The sticky bone was placed around the implants exposed surface (Figure 6), covered by two L-PRF membranes (Figure 7) and then sutured with five simple stitches using nylon threads (Shalon, 5-0 Nylon) to isolate the graft from soft tissue cells migration and support in the soft tissue healing process.
Medication
The patient medication was amoxicillin (875 mg every 12 hours for 7 days) for bacterial control, prednisolone 20 mg (1 pill an hour before surgery and 1 pill every 24 hours for 3 days) to control swelling and Nimesulide (100 mg every 12 hours for 5 days) to control inflammation.
Prosthetic management
It was waited six months after surgery for the implants osseointegration and bone formation around them to be completed before the prosthetic rehabilitation with a four elements screw-fixed prosthesis over the implants.
Case resolution
Six months after the surgery, the patient had a new panoramic radiograph to assess implants osseointegration and positioning (Figure 8).
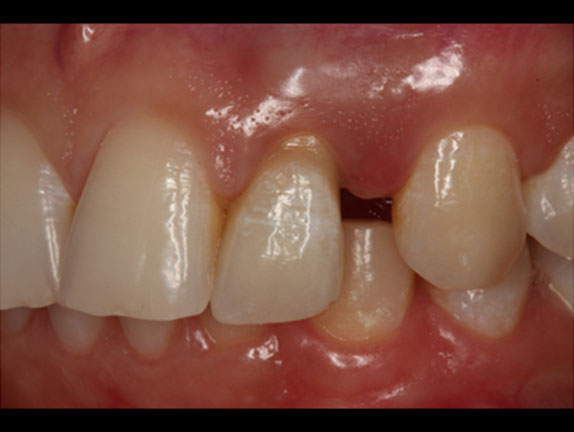
A healthy periodontal status was achieved, with good tissue thickness to help prevent future periimplantitis and grayness in the cervical area. The prosthesis needed ceramic artificial papillae between 11–12 and 21–22, to avoid black spaces because of the bone remodeling process (Figure 9).
Even though artificial papillae were needed, the patient’s low smile helped with prosthesis aesthetics, resulting in a good functional and aesthetic outcome for this case (Figure 10).
Discussion
Reabsorbed alveolar bone augmentation remains a challenging surgical procedure, especially in extensive bone atrophies. Many different augmentation techniques have been proposed to restore an adequate bone volume for implant installation, such as the guided bone regeneration technique [16].
In this case report, because of a lack of proper bone volume was diagnosed in the clinical, radiographic evaluation and implant installation, a guided bone regeneration (GBR) technique was performed. Many studies has shown that GBR has a good success outcome rate in bone height and/or thickness regeneration, using as a basic principle that the first cells that migrate to the area will define the type of tissue being regenerated [17].
Even though there is evidence of better clinical outcomes with autologous bone graft [18], tissue engineering and biomechanics have developed bone substitutes that achieve great outcomes as well. Authors like Chiapasco et al. (2006) have also used natural and synthetically materials such as hydroxyapatite, bovine bone, and a combination of them [17]. In cases reports, where xenogeneic materials were used also demonstrated favorable outcomes in the bone regenerated quality and volume [19].
In this clinical case, we decided to use a bovine bone substitute even though it has a less osteogenic potential than the autogenous bone, because of its availability and to avoid the autogenous disadvantages such as a second surgical donator site with higher patient’s morbidity and chances of post-surgical infections [20].
Kökdere et al. in 2015 performed an experimental study utilizing PRF in combination with bone grafts, showing that it increments bone formation and a positive effect on initial bone healing [21]. The sticky bone used in our case report, as principal matrix, has the presence of growth factors that accelerate the healing process and grants graft stability, which makes this an autologous graft biomaterial with the benefits of the PRF [22].
The graft area was covered with 2 L-PRF membranes as mechanical barriers, with the objective of isolate unwanted cells from migrating to the area. In the literature, different membranes and materials have been used with this purpose, such as collagen and titanium [23]. Other authors identified disadvantages in clinical outcomes with the use of reabsorbable collagen membranes such as lack of stability and accelerated reabsorption [24]. Clinical studies have been using non-reabsorbable re-enforced titanium membranes that are considered the golden standard, in combination with bone grafts for horizontal bone augmentation [25],[26].
There were also positive outcomes with the GBR technique to fill gaps around implants and the alveolar bone, with the use of sticky bone and PRF as mechanical barriers, obtaining bone formation and keratinized gingival tissue with over 2 mm thickness [15]. This technique was also used in immediate implant installation, demonstrating that the growth factors accelerate soft and hard tissue healing in the initial days [27].
We decided to use L-PRF membranes due to their versatility. They were used as a mechanical barrier to protect the graft from the oral environment and unwanted cell migration, preventing post-surgical infections by taking leucocytes to the area, as a support on cellular proliferation and inducing soft tissue regeneration with the presence of growth factors. As a result, these L-PRF membranes are capable of promoting GBR.
The technique shows good success indices, easy procedures, and less morbidity when in combination with bovine bone substitutes in comparison with conventional autologous graft techniques.
The excellent clinical outcomes in this case report corroborates the results of other authors and contributes to the establishment of a possible clinical protocol, for easy and less aggressive surgical procedures compared to conventional approaches.
Conclusion
In conclusion, the use of PRF associated with bovine bone substitutes as sticky bone and L-PRF membranes in the guided bone regeneration technique around implants contributed to the bone regeneration and soft tissue healing processes, favoring implant osseointegration over a period of six months, allowing the patients aesthetic and functional rehabilitation.
REFERENCE
1.
Strauss FJ, Stähli A, Gruber R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin Oral Implants Res 2018;29 (Suppl 18):6–19. [CrossRef]
[Pubmed]

2.
Fu YC, Nie H, Ho ML, Wang CK, Wang CH. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite Scaffolds loaded with BMP-2. Biotechnol Bioeng 2008;99(4):996–1006. [CrossRef]
[Pubmed]

3.
Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng 2014;42(2):323–37. [CrossRef]
[Pubmed]

4.
Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. Interventions for replacing missing teeth: Horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev 2009;2009(4):CD003607. [CrossRef]
[Pubmed]

5.
Athanasiou KA, Zhu C, Lanctot DR, Agrawal CM, Wang X. Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng 2000;6(4):361–81. [CrossRef]
[Pubmed]

6.
Shamsoddin E, Houshmand B, Golabgiran M. Biomaterial selection for bone augmentation in implant dentistry: A systematic review. J Adv Pharm Technol Res 2019;10(2):46–50. [CrossRef]
[Pubmed]

7.
Raghoebar GM, Batenburg RH, Vissink A, Reintsema H. Augmentation of localized defects of the anterior maxillary ridge with autogenous bone before insertion of implants. J Oral Maxillofac Surg 1996;54(10):1180–5. [CrossRef]
[Pubmed]

8.
Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res 2000;(371):10–27.
[Pubmed]

9.
Gultekin BA, Bedeloglu E, Kose TE, Mijiritsky E. Comparison of bone resorption rates after intraoral block bone and guided bone regeneration augmentation for the reconstruction of horizontally deficient maxillary alveolar ridges. Biomed Res Int 2016;2016:4987437. [CrossRef]
[Pubmed]

10.
von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: A clinical study with 42 patients. Clin Oral Implants Res 2006;17(4):359–66. [CrossRef]
[Pubmed]

11.
Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin Oral Investig 2017;21(6):1913–27. [CrossRef]
[Pubmed]

12.
Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101(3):E37–44. [CrossRef]
[Pubmed]

13.
Jang ES, Park JW, Kweon H, et al. Restoration of peri-implant defects in immediate implant installations by choukroun platelet-rich fibrin and silk fibroin powder combination graft. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109(6):831–6. [CrossRef]
[Pubmed]

14.
Chiapasco M, Casentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants 2009;24 Suppl:237–59.
[Pubmed]

15.
Zhou J, Li X, Sun X, et al. Bone regeneration around immediate placed implant of molar teeth with autologous platelet-rich fibrin: Two case reports. Medicine (Baltimore) 2019;97(44):e13058. [CrossRef]
[Pubmed]

16.
Sagheb K, Schiegnitz E, Moergel M, Walter C, Al-Nawas B, Wagner W. Clinical outcome of alveolar ridge augmentation with individualized CADCAM-produced titanium mesh. Int J Implant Dent 2017;3(1):36. [CrossRef]
[Pubmed]

17.
Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res 2006;17 Suppl 2:136–59. [CrossRef]
[Pubmed]

18.
Yang LS, Yan JW, Zheng H, Ni R, Han XK, Chang X. Comparative study of processed autogenous tooth bone and xenogeneic bovine bone in repairing an alveolar bone defect. [Article in Chinese]. Hua Xi Kou Qiang Yi Xue Za Zhi 2018;36(4):372–7. [CrossRef]
[Pubmed]

19.
Wu D, Zhou L, Lin J, Chen J, Huang W, Chen Y. Immediate implant placement in anterior teeth with grafting material of autogenous tooth bone vs xenogenic bone. BMC Oral Health 2019;19(1):266. [CrossRef]
[Pubmed]

20.
Coatoam GW, Mariotti A. The segmental ridge-split procedure. J Periodontol 2003;74(5):757–70. [CrossRef]
[Pubmed]

21.
Kökdere NN, Baykul T, Findik Y. The use of platelet-rich fibrin (PRF) and PRF-mixed particulated autogenous bone graft in the treatment of bone defects: An experimental and histomorphometrical study. Dent Res J (Isfahan) 2015;12(5):418–24. [CrossRef]
[Pubmed]

22.
Soni R, Priya A, Yadav H, Mishra N, Kumar L. Bone augmentation with sticky bone and platelet-rich fibrin by ridge-split technique and nasal floor engagement for immediate loading of dental implant after extracting impacted canine. Natl J Maxillofac Surg 2019;10(1):98–101. [CrossRef]
[Pubmed]

23.
Simion M, Jovanovic SA, Trisi P, Scarano A, Piattelli A. Vertical ridge augmentation around dental implants using a membrane technique and autogenous bone or allografts in humans. Int J Periodontics Restorative Dent 1998;18(1):8–23.
[Pubmed]

24.
Bunyaratavej P, Wang HL. Collagen membranes: A review. J Periodontol 2001;72(2):215–29. [CrossRef]
[Pubmed]

25.
Simion M, Fontana F, Rasperini G, Maiorana C. Vertical ridge augmentation by expanded-polytetrafluoroethylene membrane and a combination of intraoral autogenous bone graft and deproteinized anorganic bovine bone (Bio Oss). Clin Oral Implants Res 2007;18(5):620–9. [CrossRef]
[Pubmed]

26.
Canullo L, Malagnino VA. Vertical ridge augmentation around implants by e-PTFE titanium-reinforced membrane and bovine bone matrix: A 24- to 54-month study of 10 consecutive cases. Int J Oral Maxillofac Implants 2008;23(5):858–66.
[Pubmed]

27.
Valenzuela S, Olivares JM, Weiss N, Benadof D. Immediate implant placement by interradicular bone drilling before molar extraction: Clinical case report with one-year follow-up. Case Rep Dent 2018;2018:6412826. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Nelson Leonel Del Hierro Polanco - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Renato Martins - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gabriel Bernini - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Fernando Alonso - Conception of the work, Design of the work, Drafting the work, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Gabriela Gennaro - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
AcknowledgmentsThis work was done in the Faculdade do Centro Oeste Paulista (FACOP). There is no conflict of interest for any of the authors.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Copyright© 2021 Nelson Leonel Del Hierro Polanco et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.