|
Case Report
Pleomorphic adenoma of the soft palate: A case report
1 Lecturer at Oral and Maxillofacial Surgery Department, Faculty of Dentistry, October 6 University, Cairo, Egypt
2 Lecturer of Oral & Maxillofacial Pathology, Faculty of Dentistry, October 6 University, Cairo, Egypt
3 Associated Professor of Oral and Maxillofacial Surgery, Oral Surgery Department, Faculty of Dentistry, October 6 University, Cairo, Egypt
Address correspondence to:
Nermine Ramadan Mahmoud
Associated Professor of Oral and Maxillofacial Surgery, Oral Surgery Department, Faculty of Dentistry, October 6 University, Cairo,
Egypt
Message to Corresponding Author
Article ID: 100038Z07YH2022
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Habaka YF, El Din YA, Mahmoud NR. Pleomorphic adenoma of the soft palate: A case report. J Case Rep Images Dent 2022;8:100038Z07YH2022.ABSTRACT
Introduction: Tumors of the salivary gland represent 4% of the head and neck tumors. Pleomorphic adenoma is known as a benign mixed tumor of the salivary gland and is considered the most common benign salivary gland tumor. Interestingly, the parotid gland is considered the most common site with 80–90% and only 10% occurring in the minor salivary gland. The palate is the most prevalent place for pleomorphic adenoma in the mono salivary gland, but other sites such as the lips, cheeks, floor of mouth, tongue, tonsils, pharynx, retromolar area, and nasal cavity are also common.
Case Report: In this case, a 24-year-old Egyptian man developed a pleomorphic adenoma of the small salivary glands of the soft palate.
Conclusion: Pleomorphic adenoma, though a common entity, is still a challenging tumor for pathologists, radiologists, and surgeons. Its diverse histological and topographical property makes the tumor special. Pleomorphic adenoma could mimic other malignant lesions such as the adenoid cystic carcinoma which considered among the most common malignant salivary gland occurring at the same site.
Keywords: Adenoid cystic carcinoma, Minor salivary gland tumors, Mixed tumors, Pleomorphic adenoma (PA)
Introduction
Salivary gland tumors can be classified according to their origin, which is derived from major or minor glands. Pleomorphic adenoma (PA) is the most common benign tumor of the main salivary gland, affecting primarily the parotid gland and less frequently the minor salivary gland. It is classed as a mixed tumor, and it is responsible for 73% of all salivary gland tumors. Mixed tumors are mostly found on the palate, which correlates to small glands. The tumor, on the other hand, frequently affects the lips. On the one hand, tumors in the mouth, nose, and neck account for a modest percentage of total tumors [1],[2],[3]. On the other hand, the tonsils, tongue, pharynx, buccal mucosa, gingiva, the floor of the mouth and retromolar area are considered other intraoral sites [2].
Considering age range, pleomorphic adenoma mainly affects patients between the fourth and sixth decades, however, it can occur at any age. Pleomorphic adenoma is considered as the most common salivary gland neoplasm that occurs in children, with a percentage of 66–90% of all salivary gland tumors divided as 40% are males and 60% are females. The management was usually the wide local excision with the involved periosteum and bone [2],[3].
Regarding the clinical presentation, it usually appears as well-delineated painless swelling covered with normal mucous membrane, but sometimes, mucosal ulcerations are observed, and related nodules appear singular and mobile. Pleomorphic adenoma is usually encapsulated when occurring on major gland tumors which are opposed to minor gland tumors of being lacked well-defined capsule [3].
For histopathological presentation, PA is characterized by neoplastic proliferation of glandular cells along with myoepithelial components. The term “pleomorphic” is used to describe the histology and histogenesis of the tumor cells and derives from the Greek words for “more” and “form.” Its name came from its distinctive architectural pleomorphism, which can be detected using light microscopy. The tumor has the following three components: epithelial cell, myoepithelial cell, and stromal/mesenchymal. According to the World Health Organization (WHO), the majority of instances involve the parotid gland’s lower pole or tail. Intraoral pleomorphic adenomas are more common in the palate and submandibular glands, while they are uncommon in the sublingual glands [4].
Case Report
A 24-year-old male patient presented to Oral and Maxillofacial Surgery Department, Faculty of Dentistry, October 6 University with a slowly growing mass on the hard palate on the right side. On palpation and visual inspection, it revealed swelling in the junction of the soft and hard palate and crossing the midline, the overlying mucosa was intact non-ulcerated but bulging at places. On palpation, the mass showed a smooth surface with numerous small blood vessels, non-tender, non-fluctuant, firm, and fixed to the underlying bone with well-defined margins. No cervical lymphadenopathy. The mass did not blanch or feel pulsatile upon palpation, ruling out a vascular lesion.
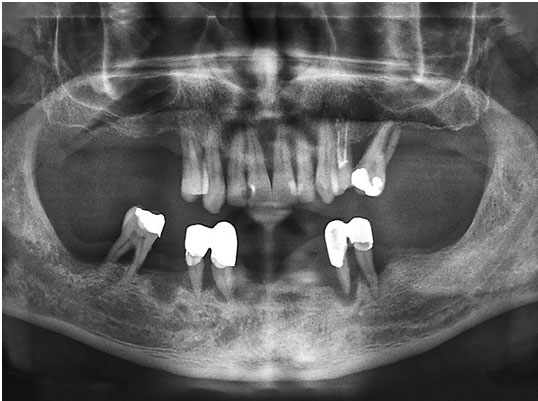
The lesion measured about 3×3 cm with no teeth involved. Computed tomography (CT) scan was done. With all these findings, the provisional diagnosis was palatal pleomorphic adenoma and the patient was scheduled for surgical excision of the lesion.
Fine needle aspiration cytology (FNAC) was performed using 22–23 gauge needle under septic conditions, smears were performed and slides were stained with H&E.
Surgical procedure
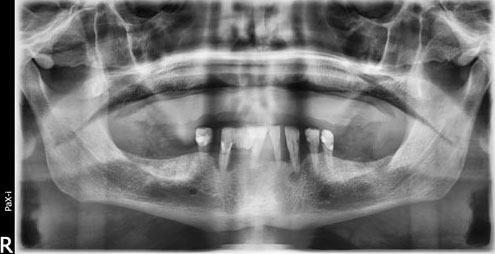
The patient was scheduled for surgical excision under local anesthesia. A mucosal incision was made atop the lesion, then a wide dissection was performed, and the entire encapsulated tumor mass, as well as the mucoperiosteum, was removed with 1 cm safety margins and with meticulous dissection (Figure 1). Watertight surgical wound closure was largely accomplished in layers with mucosal advancement (Figure 2).
The excised mass was prepared for histopathological examination. Tissue for histopathologic analysis was rapidly dissected and fixed in 10% buffered formalin. The tissues were embedded in paraffin and 5 μm sections were cut. Sections were stained with Hematoxylin and Eosin (H&E). H& E slides were prepared to confirm the diagnosis using light microscope examination.
The patient’s postoperative recovery went smoothly. Postoperative instructions and antibiotic were: Augmentin (1 g every 12 hours/5 days), Dexamethasone (8 mg/2 mL Ampoule I.M., every 12 hours for 3 days), Voltaren (75 mg/3mL Ampoule I.M. every 12 hours for 3 days).1
Postoperative follow-up, weekly for the first month then monthly for 1 year (Figure 3).
In the first week, granulation tissue was formed, then healing was uneventful, no postoperative uvula deviation, no speech problem nor deglutition difficulty. No recurrence was observed after one year follow-up.
Histopathological examination
Histopathological examination revealed a pseudocapsule surrounding mass showing proliferating glandular epithelial cells exhibiting architectural diversity which is a characteristic feature for PA. The encapsulated tumor growth is composed of a mixture of epithelial and myoepithelial/stromal components. Epithelial elements are disposed of in ducts lined by a layer of cuboidal cells lying on the attuned myoepithelial cell layer. Spindled myoepithelial cells stream from the ductal elements into the stroma. Some ducts contain eosinophilic secretion within their lumina and some with hyperemia of the stromal vessels are present. The stromal elements are composed of hyalinized stroma with few chondromyxoid areas. Areas of blood spaces are scattered among the lesions. No abnormal or dysplastic features were noticed at the margins during microscopic examination of the specimen (Figure 4A, Figure 4B, Figure 4C, Figure 4D, Figure 4E, Figure 4F).
1gsk GlaxoSmithKline (875 amoxycillin + 125 clavulonic postassium)
AMRIYA PHARM IND NOVARTIS (diclofenac sodium 75 mg/3 mL)
Discussion
Tumors of the salivary gland account for less than 4% of all head and neck cancers. It usually occurs during the fourth and sixth decades, however, it has been observed in patients as young as 7 years old and as elderly as 82 years old [5]. Pleomorphic adenoma was found in the parotid gland in 70–84% of cases, the submandibular gland in 8% of cases, and the minor salivary glands in only 4–6% of cases [6].
Many surgical approaches are possible for soft palate tumors treatment including transcervical, mandibulotomy, mandibular swing, and intraoral approaches. The choice of surgical approach depends on tumor size and its location, transmandibular approach was deemed necessary when it extended from the soft palate to the parapharyngeal space to allow good exposure, ensuring its complete excision, as well as control any feeding vessels to prevent hemorrhage. Five factors should be considered when deciding on the best surgical method for removing a soft palate and parapharyngeal space tumor was suggested by [7], they are as follows: (1) Tumor size, (2) Projection of tumor and its proximity to oropharyngeal wall, (3) Suspected malignancy, (4) Vascularity, and (5) Relation of the tumor to the neck neurovascular bundle.
Mendenhall et al. (2008) reported in their study that the best management approach of palatal PAs was the wide excision with negative margins especially the malignant transformation that has been described at a rate of 1.9–23.3% [8],[9]. Although, the recurrence of tumors occurring in the minor salivary gland is considered uncommon [10]. However, it was increased by intraoperative spillage of the tumor and inadequate excision [11]. For radiotherapy, it was reserved for recurrent tumors or inoperable.
Careful diagnostic investigations preoperatively were mandatory because of the higher risk of malignancy [12]. In their study they recommended CT scanning for assessment of bony erosions, which indicated the more aggressive pattern of the lesion. They also suggested that magnetic resonance imaging (MRI) which was useful in submucosal lesions diagnosis such as pleomorphic adenoma as they return characteristically low in T1-weighted signal and high in T2-weighted signal, often with a low-signal fibrous capsule. Moreover, MRI can provide excellent soft tissue definition and detect perineural spread.
Furthermore, Patel et al. (2015) reported in their study that anesthetic considerations were mandatory when managing intraoral minor salivary gland tumors (IMSGT) of the palate. Due to tumor extension, the endotracheal and fiberoptic nasal intubation was not an option; therefore, the decision to make a tracheostomy was made. Transnasal humidified rapid insufflation ventilatory exchange (THRIVE) was used during tracheostomy. It consists of high flow humidified nasal oxygen therapy, delivered at up to 70 L/min, using Opti Flow. In individuals with difficult airways, it has been proven to sustain oxygen saturations and lengthen apnoea time [13]. It has proven to be a valuable addition to the awake tracheostomy procedure in a previous local anesthetic tracheostomy [14], and it was proved similarly effective in their case.
Pleomorphic adenoma’s recurrence can be considered as multi-factorials. So, intraoperative rupture of the tumor capsule and incomplete tumor excision, especially in cases of large parotid tumors involving the facial nerve can be considered an important factor [15] as well as the size of the surgical margin [16]. Moreover, the duration of the tumor before initial treatment was considered also as a risk factor. The main problem with recurrence is the increased risk of malignant transformation, which is estimated at 5–20% [17]. The WHO salivary gland tumor experts estimate that about 12% of carcinoma PA cases develop from recurrent PA tumors [18].
Pleomorphic adenoma is characterized by complex structure and diversity in microscopic appearance when considering histological features. A mixture of polygonal epithelial and spindle-shaped myoepithelial elements, as well as a variety of background stroma, which can be myxoid, cartilaginous, mucoid, or hyaline, give it a biphasic look. Moreover, epithelial components can also be arranged in clusters, sheets, duct-like or interlacing threads, with cells that are spindle, polygonal, or stellate shaped. Furthermore, epithelial pearls and squamous metaplasia may occur in rare situations. The tumor was surrounded but not enclosed by a fibrous pseudo-capsule [19].
Conclusion
Pleomorphic adenoma, though a common entity, is still a challenging tumor for pathologists, radiologists, and surgeons. Its diverse histological and topographical property makes the tumor special. Pleomorphic adenoma could mimic other malignant lesions such as the adenoid cystic carcinoma which considered among the most common malignant salivary gland occurring at the same site, also PA resembles other more innocuous lesions such as lipoma and be missed on a CT scan, hence MRI was recommended because of the high-fat content. Obtaining the definitive diagnosis in aid of histopathological examination was especially important since long-standing pleomorphic adenomas have the potential to become malignant, early detection and tumor excision with safety margins is well recommended.
REFERENCE
1.
Passi D, Ram H, Dutta SR, Malkunje LR. Pleomorphic adenoma of soft palate: Unusual occurrence of the major tumor in minor salivary gland—A case report and literature review. J Maxillofac Oral Surg 2017;16(4):500–5. [CrossRef]
[Pubmed]

2.
3.
Shrestha A, Reddy N, Ganguly S. Pleomorphic adenoma of the upper lip: A case report. Journal of College of Medical Sciences-Nepal 2010;6(1):51–3. [CrossRef]

4.
Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging 2007;7(1):52–62. [CrossRef]
[Pubmed]

5.
Luna MA, Batasakis JG, el-Naggar AK. Salivary gland tumors in children. Ann Otol Rhinol Laryngol 1991;100(10):869–71. [CrossRef]
[Pubmed]

6.
Dhanuthai K, Sappayatosok K, Kongin K. Pleomorphic adenoma of the palate in a child: A case report. Med Oral Patol Oral Cir Bucal 2009;14(2):E73–5.
[Pubmed]

7.
Papadogeorgakis N, Petsinis V, Goutzanis L, Kostakis G, Alexandridis C. Parapharyngeal space tumors: Surgical approaches in a series of 13 cases. Int J Oral Maxillofac Surg 2010;39(3):243–50. [CrossRef]
[Pubmed]

8.
Mendenhall WM, Mendenhall CM, Werning JW, Malyapa RS, Mendenhall NP. Salivary gland pleomorphic adenoma. Am J Clin Oncol 2008;31(1):95–9. [CrossRef]
[Pubmed]

9.
Ethunandan M, Witton R, Hoffman G, Spedding A, Brennan PA. Atypical features in pleomorphic adenoma—A clinicopathologic study and implications for management. Int J Oral Maxillofac Surg 2006;35(7):608–12. [CrossRef]
[Pubmed]

10.
Hickman RE, Cawson RA, Duffy SW. The prognosis of specific types of salivary gland tumors. Cancer 1984;54(8):1620–4. [CrossRef]
[Pubmed]

11.
Kuo YL, Tu TY, Chang CF, et al. Extra-major salivary gland pleomorphic adenoma of the head and neck: A 10-year experience and review of the literature. Eur Arch Otorhinolaryngol 2011;268(7):1035–40. [CrossRef]
[Pubmed]

12.
von Stempel C, Morley S, Beale T, Otero S. Imaging of palatal lumps. Clin Radiol 2017;72(2):97–107. [CrossRef]
[Pubmed]

13.
Patel A, Nouraei SAR. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): A physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia 2015;70(3):323–9. [CrossRef]
[Pubmed]

14.
Abeysundara L, Parker H, Fowler A, Patel A. The use of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) to facilitate tracheostomy under sedation. Anaesthesia Cases 2016;4(1):17–9. [CrossRef]

15.
Kici?ski K, Mikaszewski B, Stankiewicz C. Risk factors for recurrence of pleomorphic adenoma. Otolaryngol Pol 2016;70(3):1–7.
[Pubmed]

16.
Riad MA, Abdel-Rahman H, Ezzat WF, Adly A, Dessouky O, Shehata M. Variables related to recurrence of pleomorphic adenomas: Outcome of parotid surgery in 182 cases. Laryngoscope 2011;121(7):1467–72. [CrossRef]
[Pubmed]

17.
Maxwell EL, Hall FT, Freeman JL. Recurrent pleomorphic adenoma of the parotid gland. J Otolaryngol 2004;33(3):181–4.
[Pubmed]

18.
El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumors (4th ed, Vol. 9), IARC, WHO: Lyon. 2017. [Available at: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Head-And-Neck-Tumours-2017]

19.
Hellquist H, Paiva-Correia A, Poorten VV, et al. Analysis of the clinical relevance of histological classification of benign epithelial salivary gland tumours. Adv Ther 2019;36(8):1950–74. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Yasser Fekry Habaka - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Yasmine Alaa El Din - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Nermine Ramadan Mahmoud - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2022 Yasser Fekry Habaka et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.