|
Case Report
Hormonal influence on adenomatoid odontogenic tumor: A case report in pregnancy
1 MDS Oral Medicine & Radiology, Mumbai, India
2 Associate Professor, Department of Oral Medicine & Radiology, Nair Hospital Dental College, Mumbai, India
3 Assistant Professor, Department of Oral Medicine & Radiology, Government Dental College & Hospital, Aurangabad, India
4 Postgraduate, Department of Oral Medicine & Radiology, Nair Hospital Dental College, Mumbai, India
Address correspondence to:
Shivani Singh
MDS Oral Medicine & Radiology, Mumbai,
India
Message to Corresponding Author
Article ID: 100050Z07SS2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Singh S, Vahanwala S, Malu P, Soni S. Hormonal influence on adenomatoid odontogenic tumor: A case report in pregnancy. J Case Rep Images Dent 2025;11(1):11–17.ABSTRACT
Introduction: Pregnancy is a delicate physiologic state accompanied by increased risk of pathologic exposure. Oral complications like gingival enlargements, epulis, etc. are commonly seen in pregnant women owing to immune and hormonal changes. Although very few cases of odontogenic tumors have been reported, many authors have reported major estrogen-dependent tumors presumed to arise due to differences in genetic expression. Similarly, the influence of hormones in the growth of odontogenic tumors is also postulated. The correlation of certain proteins and receptors is being reviewed to understand the pathophysiology of potential interactions between hormones and odontogenic tumor cells. The literature search was conducted with the aim to derive a vivid explanation by identifying a common component between pregnancy and the occurrence of head and neck tumors.
Case Report: A report of patient in third trimester of pregnancy presenting with an odontogenic tumor, followed by a review of the possible relation between hormone and occurrence of odontogenic tumors in pregnant women.
Conclusion: Understanding the relationship between hormonal levels in females and the occurrence of jaw tumors will emphasize on importance of screening and follow-up of oral cavity in pregnant population and appropriate referral to a specialist, if necessary.
Keywords: Adenomatoid odontogenic tumor, Bcl-2, Estrogen receptor, Odontogenic tumor, Pregnancy
Introduction
Pregnancy, a physiological phase in a woman’s life, extensively alters the blood chemistry to nurture the developing fetus. During this time, utmost care for the mother directly or indirectly ensures a safer environment for the fetus and a better survival rate after birth. Hormonal variations are considered “prima facie” evidence for the numerous oral changes that have been reported in the literature [1].
Oral mucosal lesions associated with pregnancy are well documented; additionally, the occurrence of benign head and neck tumors in pregnant individuals has been mentioned, but such data remain sparse [2]. Tumors reported during pregnancy have conventional clinical and radiographic findings, interestingly though the tumors exhibit rapid growth rate when the patient is pregnant or during gestation. This suggests that the proliferation rate of odontogenic tumors during pregnancy may be accelerated, but the exact mechanism and possible correlation between hormonal variation on tumor growth and development is yet to be understood [3]. Radiographic exposure and surgical intervention may have to be delayed during pregnancy, this in turn can worsen the prognosis [4]. Hence having a better understanding of the hormone-tumor relationship in such patients can be crucial.
The aim of this review was to assess the pattern of growth of tumors during the phases of pregnancy and uncover the underlying mechanism by exploring the basic components common to pregnancy and the occurrence of head and neck tumors.
Case Report
A female patient in her twenties, reported to the Department of Oral Medicine and Radiology with swelling on the left side of her face, localized to the middle third, which had been evident for two months. The patient was in the third trimester of pregnancy and reported pain associated with the swelling. The pain aggravated on mastication. There was no history of trauma to the face or upper front teeth and no events of extra-oral or intra-oral pus discharge. The patient did not receive any dental treatment previously.
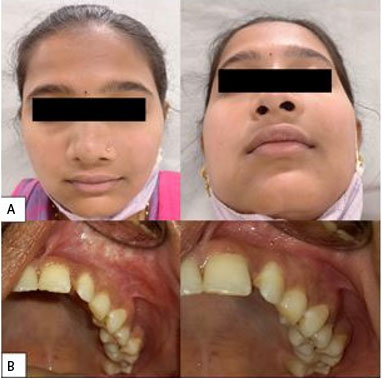
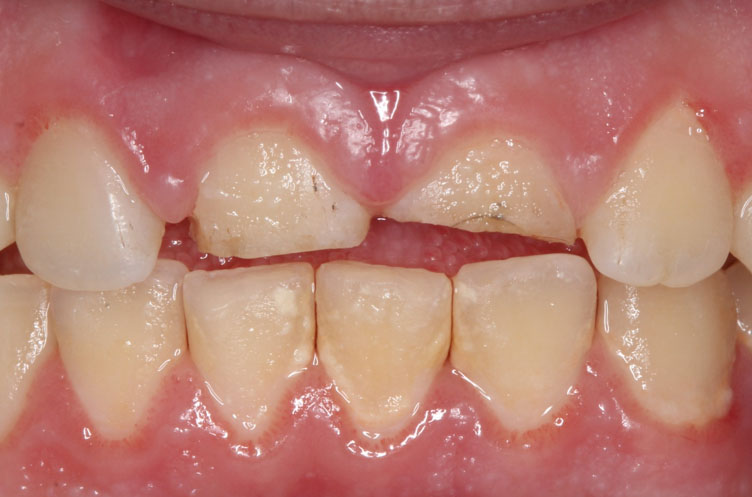
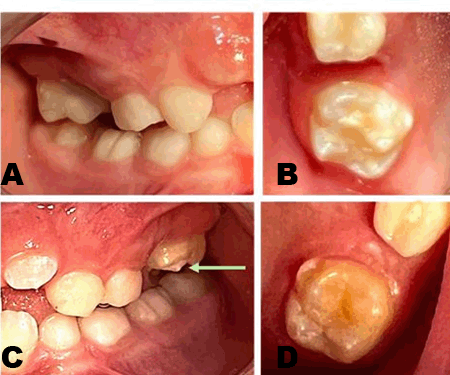
Clinically, a diffuse, firm swelling was noted on the left side of the face, lateral to the nose, with obliteration of the left nasolabial fold and raised left ala of the nose. There was no discoloration of the overlying skin and no signs of extra-oral drainage. On palpation, tenderness was present but there was no rise in local temperature. Intra-orally, a smooth, round, firm, swelling was palpable, extending from the maxillary canine to the second premolar region, causing obliteration of the buccal vestibule. A deciduous left maxillary canine was present while the permanent left maxillary canine was absent. There was no evidence of displacement of associated teeth. The patient had good overall oral hygiene, with no other gingival or mucosal lesions (Figure 1).
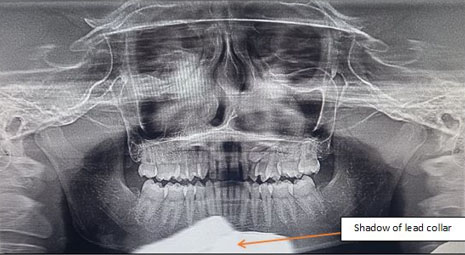
On her previous visit to a local clinic, she was prescribed a panoramic radiograph, which she carried with herself. A panoramic radiograph presents a tenfold reduction in the amount of radiation exposure and limits the dose absorbed by the patient compared to periapical radiography. For pregnant women, since the intrauterine dose absorbed is less than 10-6 rad, the risk of mental abnormalities due to dental radiology is considered nonexistent. Since patient was in the third trimester of pregnancy and active immediate intervention was not planned, computed tomography (CT) was not recommended till a later date.
Panoramic radiograph revealed a well-defined unilocular expansile non-homogenous radiolucent lesion with smooth corticated borders, extending in the left maxillary sinus and left side of the nasal cavity. Internally, the pathology was associated with the radicular portion of the impacted canine of the second quadrant. The coronal portion of the impacted maxillary canine was noted distal to the radicular portion of maxillary lateral incisor, and the radicular portion in proximity to the roots of the maxillary premolars suggesting horizontal impaction. Superior displacement in floor of left maxillary sinus was noted, thereby causing reduction in sinus dimensions. Left lateral wall of the nasal cavity was not traceable. Over-retained deciduous maxillary left canine was present with evidence of external root resorption. Since the pathology was associated with impacted tooth/maxillary canine, an obvious diagnosis of adenomatoid odontogenic tumor (AOT) was considered. The inferior most part of the radiograph showed a diffuse well-defined radiopaque shadow in midline suggestive of shadow of thyroid/lead collar (Figure 2).
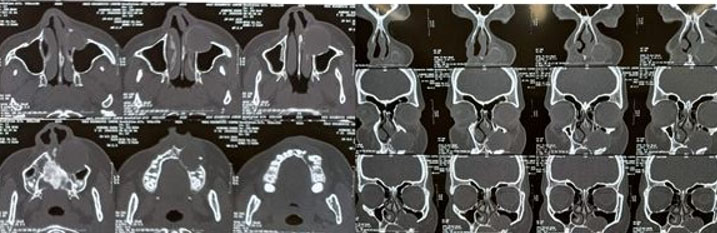
The patient was in the third trimester; therefore, surgery was planned for a later date. Pre-operative CT scan revealed a well-defined unilocular expansile non-homogenous hypodense area with a well-defined corticated border extending from the periapical region of 22–26 (anteroposteriorly) and from the alveolar bone to the maxillary sinus (superoinferiorly). Internal architecture showed the presence of a well-defined tooth-like structure along the inferior aspect, most likely to be a horizontally impacted maxillary canine. Medial displacement of the lateral nasal wall was noted on the left side. Superior displacement of floor of maxillary sinus was noted on left side, causing reduction in sinus volume of left maxillary sinus. Displacement of buccal cortical plate was noted in relation to the lesion suggestive of cortical expansion. Some areas of discontinuity were noted in the buccal cortical plate suggestive of perforation. There was no evidence of external root resorption with maxillary left canine and premolars. Computed tomography examination gave a clearer picture of the extent of the lesion for appropriate surgical planning (Figure 3).
On surgical excision, the tissue was sent for histopathology. Final histopathological evaluation revealed it to be an adenomatoid odontogenic tumor. Although there is a known female predilection for AOT, in this case the tumor became evident during pregnancy, suggesting that estrogen or progesterone might play some role in the development or rapid growth of the lesion.
Discussion
Oral mucosal lesions that arising during the period of gestation are complex and attributable to immune and hormonal changes. Such modifications in hormonal levels bring about secondary changes in the physiology of somatic tissues, potentially revealing pre-existing disorders or causing development of new diseases [5]. The principal hormones involved in the reproductive development of females are estrogen and progesterone. Estrogens are not only responsible for functioning of the female reproductive system and development of secondary sexual characteristics but also play vital role in wide range of physiological functions, including regulation of the menstrual cycle, reproduction, bone density, brain function, cholesterol mobilization, development of breast tissue and sexual organs, and control of inflammation.
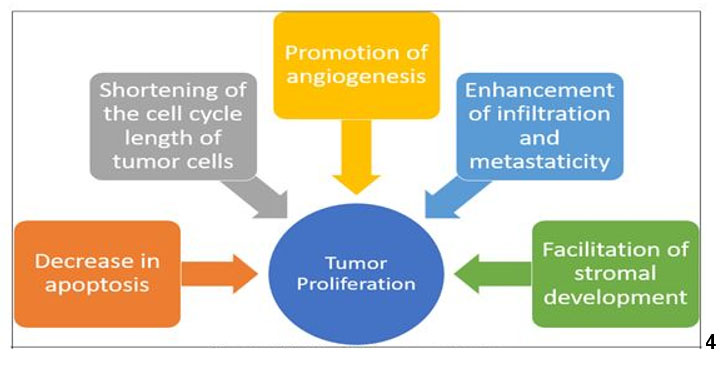
Initial studies conducted for exploring the mechanism of action of estrogen on target tissues, suggested that estrogen binds to a protein in its target cell and has cascading actions thereafter. Elwood Jensen played a pivotal role in the discovery of estrogen receptor and demonstrated that estrogen-bound receptors were able to migrate to the nucleus, thus stimulating gene transcription. Each hormone exerts their action by binding to specific receptors, which in turn activate transcriptional processes and/or signaling events that result in the control of gene expression [6],[7]. Research has linked estrogen to several major estrogen-dependent tumors, such as breast cancer, endometrial cancer, and ovarian cancer (Figure 4). Additionally, some brain tumors, including meningioma, pituitary adenoma, and glioblastoma tumors, have been reported that they are not only associated with sex hormones but also enlarge significantly during pregnancy [8],[9],[10]. This suggests that tumor growth in the presence of elevated estrogen levels may not be limited to estrogen-receptive tumors. Although there is an existing link between breast cancer and pregnancy, Bradley highlighted the point that tissues in the head and neck region lack these hormonal receptors. He suggested that the altered immunologic mechanism designed for fetal survival, may be responsible for advancement of tumor or malignancy [11].
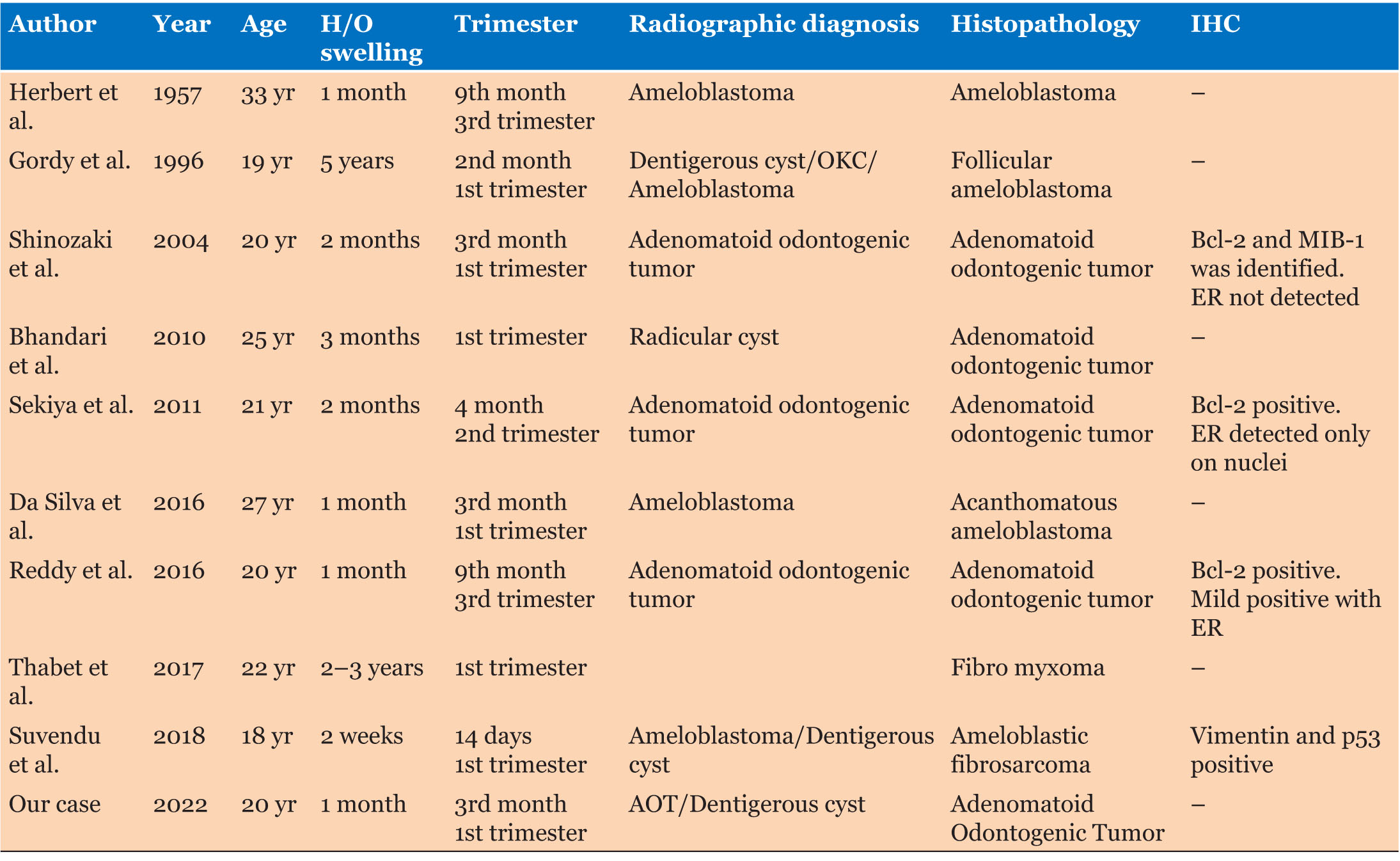
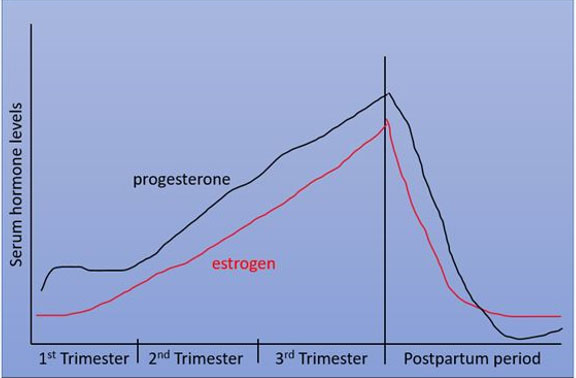
A literature search revealed about nine such case reports of odontogenic tumors in the intra-gestational phase (Table 1) [2],[3],[12],[13],[14],[15],[16],[17],[18]. Figure 5 shows the serum hormonal variation of estrogen and progesterone with proportionate surge in the levels of each, during the first trimester and peaking serum levels in the third trimester. Analysis of Table 1 highlights that most of the tumors were detected in the first trimester, while two cases were reported or diagnosed in the third trimester, with a history of the swelling of less than a month. The occurrence or detection of tumor at these specific gestational milestones is concurrent with the increase in serum hormonal levels as seen in Figure 5. This proposes a possible impact of hormones on not necessarily the initiation, but at least the rapid progression of odontogenic tumors during pregnancy.
Immunohistochemistry (IHC) is a powerful tool that exploits the specific antibody-antigen binding to detect and localize specific antigens in cells and tissue, commonly using a light microscope [19]. The authors have evaluated the existence of estrogen receptors and some other proteins. One such protein is Bcl-2, which is a CREB-related gene product, which inhibits cell apoptosis and plays an important role in tumor cell survival. Molecular research suggests that the bcl-2 gene exhibits an estrogen responsive element (ERE) in its coding region. Though the pathological function of Bcl-2 is not clear, enhanced expression of Bcl-2 has been identified in other malignancies, particularly in estrogen receptor positive (ER+) breast cancer, where it is considered a favorable prognostic marker and a target for therapy. In a case report, Sekiya et al. suggested that tumor cell survival, facilitated by Bcl-2 upon continuous stimulation by estrogen, resulted in the enlargement of the tumor during pregnancy. Expression of Bcl-2 protein has been detected in epithelial tumor cells, but it was neither evident in squamous cell carcinoma, which originated from the oral cavity, nor in normal mucosal epithelium. Case reports by Shinozaki et al., Sekiya et al., and Reddy et al. showed IHC outcome positive for Bcl-2 with no evidence of ER (estrogen receptor) [3],[13],[15].
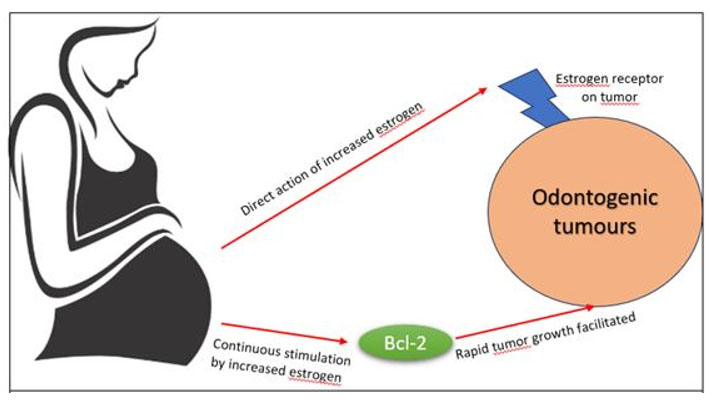
Based on these findings, two schools of thought can be proposed for the odontogenic change in the jaws in coordination with gestational hormonal alteration (Figure 6).
- The presence of estrogen receptors in tumor cells, wherein estrogen would supposedly boost the proliferation of the endogenous cells.
- Secondly, rapid tumour growth is facilitated by other proteins, which undergo continuous stimulation by estrogen.
Further research with IHC as a diagnostic modality needs to be considered in all such cases to accumulate strong evidence correlating pregnancy to odontogenic tumors. Exploring the presence of hormonal receptors in odontogenic epithelium, oral epithelium, and odontogenic lesions can pave the way for understanding gender predilection of odontogenic pathologies.
Conclusion
In the era of active health awareness by society, several patients and health professionals still remain apprehensive regarding dental evaluation in the gestational period.
This review suggests a possible correlation between the occurrence of an odontogenic tumor and pregnancy. Although physiologic phases appear to be non-relevant, as clinicians’ utmost vigilance has to be kept at such physiologic phases when surges in hormones can lead to expansile or fulminating lesions to be tackled after the cessation of the hormonal surge postpartum.
REFERENCE
1.
Bett JVS, Batistella EÂ, Melo G, et al. Prevalence of oral mucosal disorders during pregnancy: A systematic review and meta-analysis. J Oral Pathol Med 2019;48(4):270–7. [CrossRef]
[Pubmed]

2.
Gordy FM, Holder R, O’Carroll MK, Krolls SO. Growth of an ameloblastoma during pregnancy: Opportunity lost? Spec Care Dentist 1996;16(5):199–203. [CrossRef]
[Pubmed]

3.
Sekiya R, Yamazaki H, Izawa K, Kaneko A, Tsukinoki K. Case of adenomatid odontogenic tumor during pregnancy. Tokai J Exp Clin Med 2011;36(4):124–7.
[Pubmed]

4.
Gamba TO, Visioli F, Bringmann DR, Rados PV, da Silveira HLD, Flores IL. Impact of dental imaging on pregnant women and recommendations for fetal radiation safety: A systematic review. Imaging Sci Dent 2024;54(1):1–11. [CrossRef]
[Pubmed]

5.
Gangakhedkar GR, Kulkarni AP. Physiological changes in pregnancy. Indian J Crit Care Med 2021;25(Suppl 3):S189–92. [CrossRef]
[Pubmed]

6.
Jensen EV, Jordan VC. The estrogen receptor: A model for molecular medicine. Clin Cancer Res 2003;9(6):1980–9.
[Pubmed]

7.
Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 2019;116:135–70.
[Pubmed]

8.
Whitaker SB, Bouquot JE, Alimario AE, Whitaker TJ Jr. Identification and semiquantification of estrogen and progesterone receptors in pyogenic granulomas of pregnancy. Oral Surg Oral Med Oral Pathol 1994;78(6):755–60. [CrossRef]
[Pubmed]

9.
Grimm M, Biegner T, Teriete P, Hoefert S, Krimmel M, Munz A, Reinert S. Estrogen and progesterone hormone receptor expression in oral cavity cancer. Med Oral Patol Oral Cir Bucal 2016;21(5):e554–8. [CrossRef]
[Pubmed]

10.
Gissi DB, Gabusi A, Tarsitano A, Rossi R, Balbi T, Morandi L. Multi-region sequence analysis of a pregnancy-related oral squamous cell carcinoma exhibiting low-level aggressive behavior. Int J Surg Pathol 2020;28(2):188–95. [CrossRef]
[Pubmed]

11.
Bradley PJ, Raghavan U. Cancers presenting in the head and neck during pregnancy. Curr Opin Otolaryngol Head Neck Surg 2004;12(2):76–81. [CrossRef]
[Pubmed]

12.
Herberts G, Sandstrom J. Ameloblastoma occurring and recurring during pregnancies. Acta Otolaryngol 1957;48(4):327–32. [CrossRef]
[Pubmed]

13.
da Silva HEC, Costa Edo SR, Medeiros ACQ, Pereira PSDS. Ameloblastoma during pregnancy: A case report. J Med Case Rep 2016;10(1):244. [CrossRef]
[Pubmed]

14.
15.
Bhandari N, Kothari M. Adenomatoid odontogenic tumour mimicking a periapical cyst in pregnant woman. Singapore Dent J 2010;31(1):26–9. [CrossRef]
[Pubmed]

16.
Reddy GSP, Reddy GV, Reddy NVSS, Reddy KSK, Sulthana R, Phanitej G, Jyothi ED. Adenomatoid odontogenic tumor in pregnancy: A case report. Int J Case Rep Images 2016;7(10):633–9. [CrossRef]

17.
Alhousami T, Sabharwal A, Gupta S, Aguirre A, Park E, Kramer JM. Fibromyxoma of the jaw: Case report and review of the literature. Head Neck Pathol 2018;12(1):44–51. [CrossRef]
[Pubmed]

18.
Maji S, Ghosh I. Ameloblastic fibrosarcoma in pregnancy: An Unreported entity! Indian J Surg Oncol 2019;10(1):180–3. [CrossRef]
[Pubmed]

19.
Magaki S, Hojat SA, Wei B, So A, Yong WH. An introduction to the performance of immunohistochemistry. Methods Mol Biol 2019;1897:289–98. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Shivani Singh - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sonal Pratapsingh Vahanwala - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Pooja Malu - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sakshi Soni - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Shivani Singh et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.